in medical puncture devices
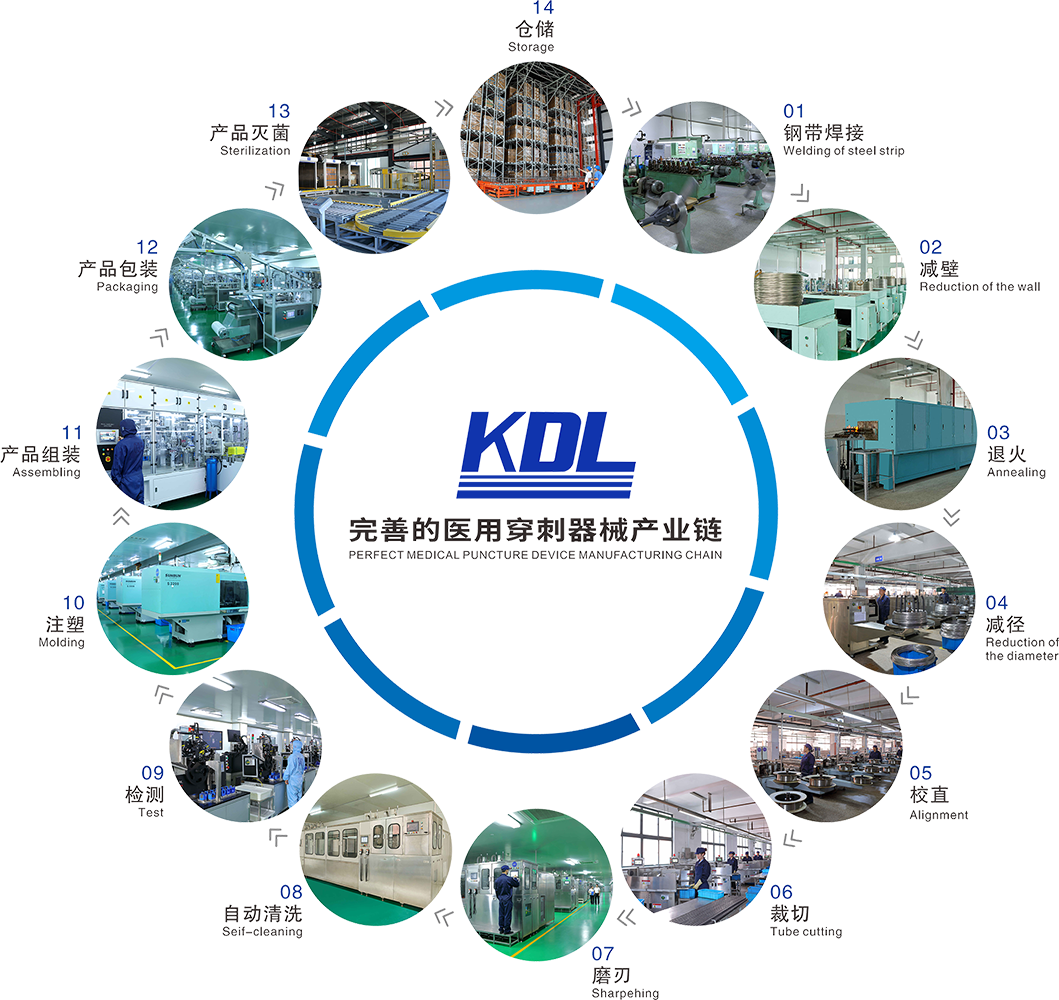
Shanghai Kindly Enterprise Development Group Co., Ltd (hereinafter referred to as the "Company" or "KDL", stock code: 603987) was founded in Wenzhou in 1987 and has expanded its operations into the Yangtze River Delta region, the Beijing-Tianjin-Hebei metropolitan economic zone, and the Guangdong-Hong Kong-Macao Greater Bay Area. The Company specializes in the research, development, production, and sales of single-use medical puncture instruments. As a leading enterprise in China's medical puncture needle manufacturing technology, it is one of the few domestic manufacturers possessing a complete industrial chain for medical puncture instruments. For multiple consecutive years, the Company has served as Vice President Unit of the China Medical Devices Industry Association and Vice Chairman Unit of the Polymer Products Branch of the China Medical Devices Industry Association.