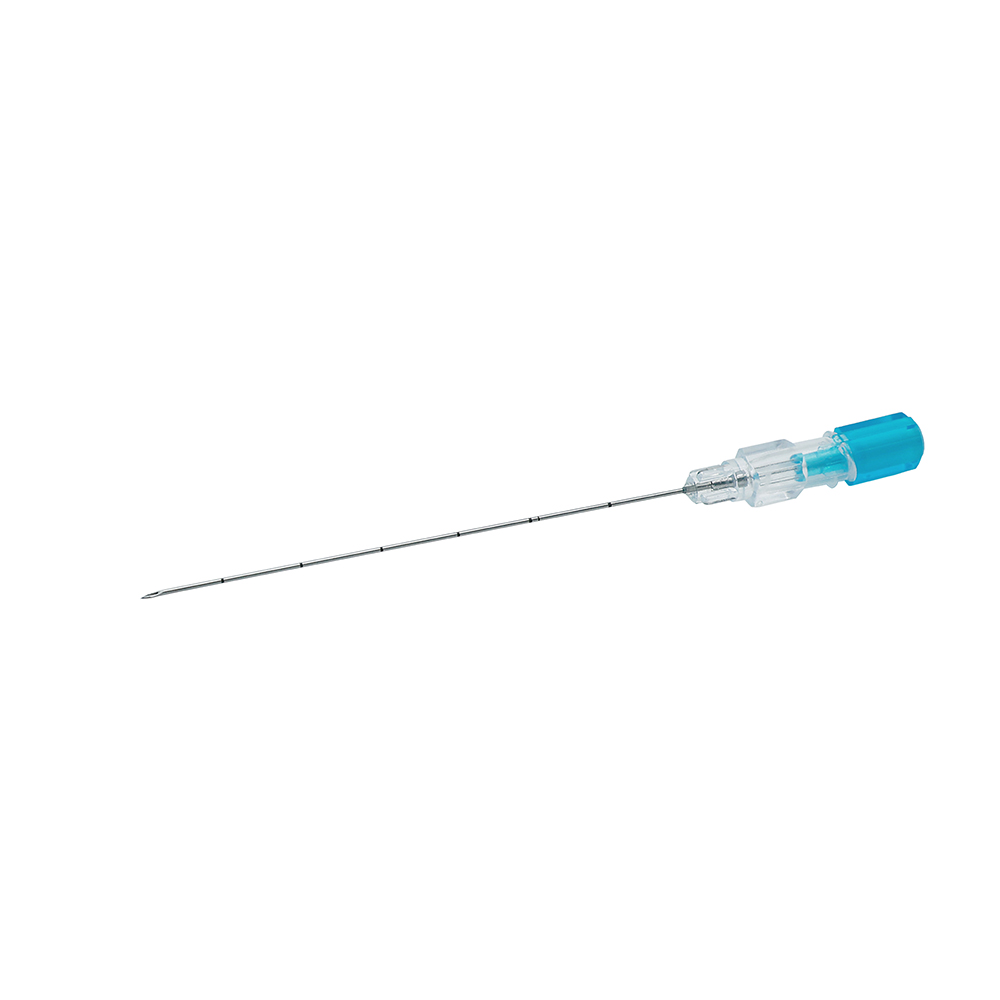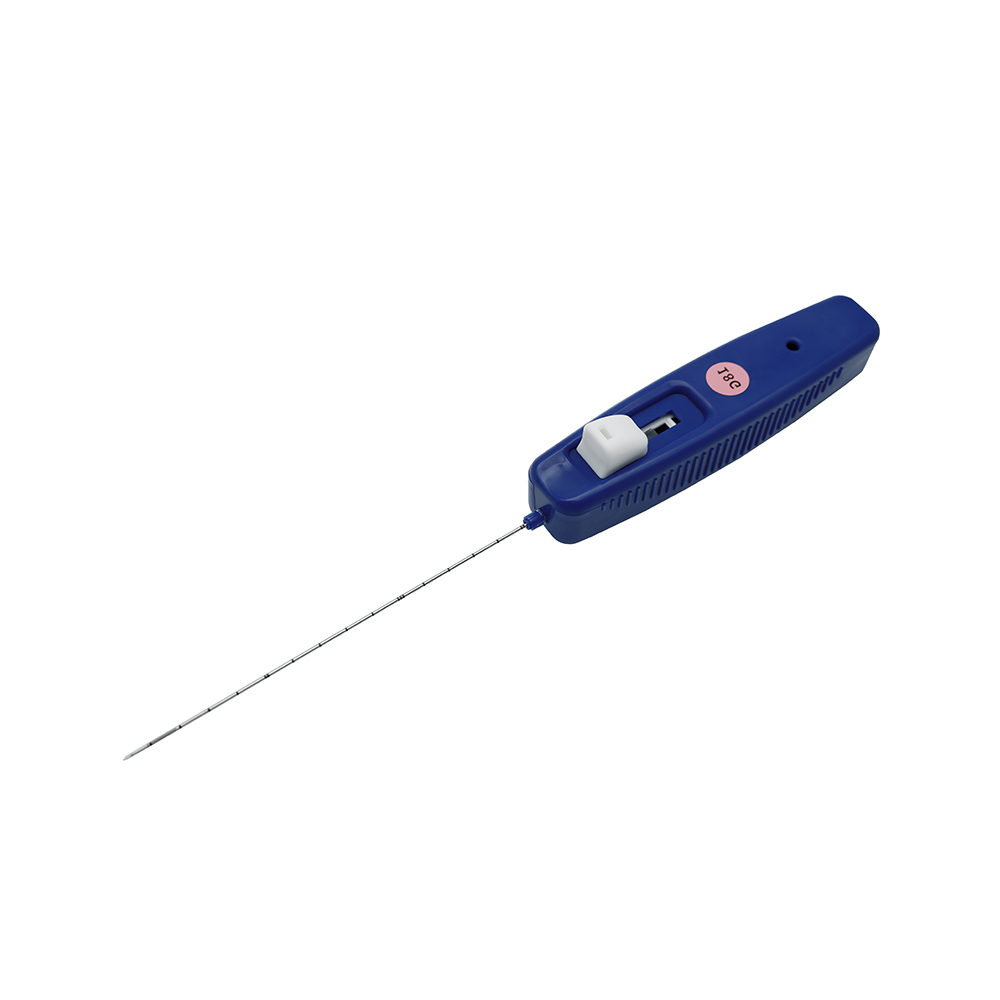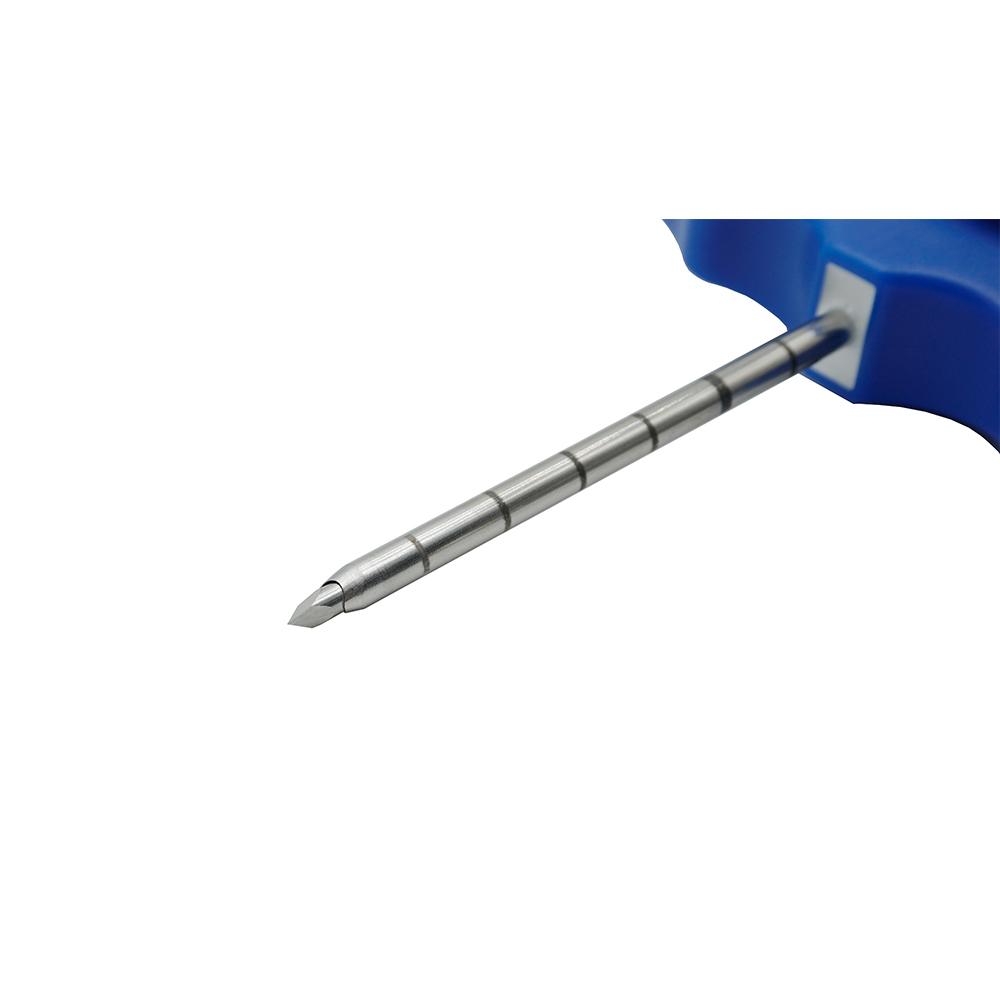

MINIMALLY INVASIVE BONE MARROW BIOPSY ASSEMBLY
Overview:
● 7-18G, needle length 68mm-150mm, regular wall
● Sterile, non-pyrogenic, sharp needle point
● Luer lock/compatible connectors for device attachment
● Sterile, non-pyrogenic, sharp needle point
● Luer lock/compatible connectors for device attachment
Product Features
| Intended use | The minimally invasive bone marrow biopsy needle kit is a sterile, single-use medical device intended for use by qualified clinicians. It is designed to obtain bone marrow core tissue specimens through a minimally invasive approach, facilitating the diagnosis, staging, and monitoring of hematological disorders (such as leukemia, myelodysplastic syndromes, and multiple myeloma) as well as evaluating bone marrow involvement in systemic diseases. The kit enables precise penetration into the bone marrow cavity at standard puncture sites (including iliac crest, sternum with appropriate precautions, and tibia in pediatric patients) with reduced tissue trauma, providing high-quality histological samples for pathological examination. It is not intended for use in non-marrow bone procedures, soft tissue biopsies, or administration of therapeutic agents, and must be used in accordance with sterile techniques and proper medical waste disposal protocols after single use. |
| Structure and composition | Stylet hub, stylet (sharp or blunt), needle tube, needle hub and protective cap, scalp marking. |
| Main Material | PP, ABS, SUS304 Stainless Steel Cannula, Silicone Oil |
| Shelf life | 5 years |
| Certification and quality assurance | Manufacturing site is in compliance with ISO 13485 and ISO 9001 Quality System |
Product Introduction









 Home
Home