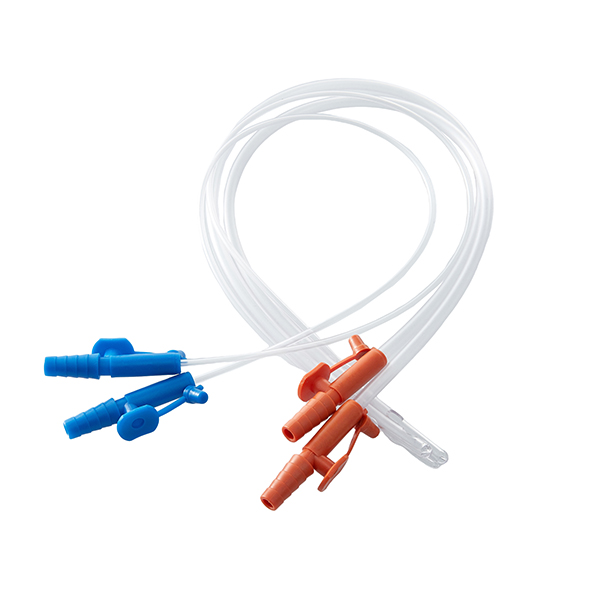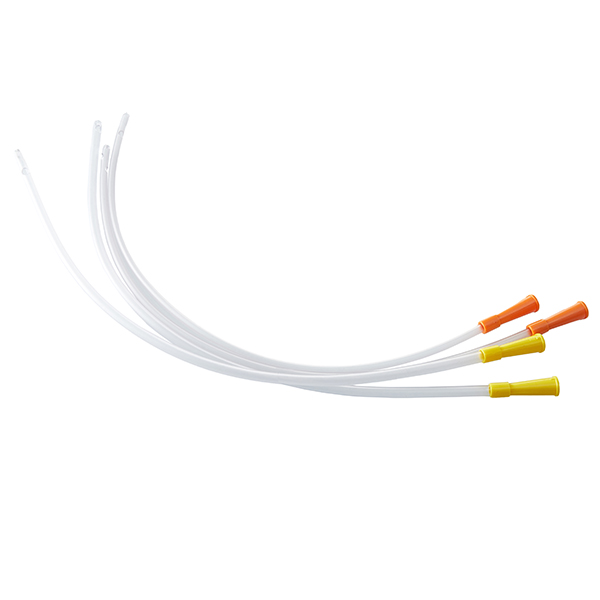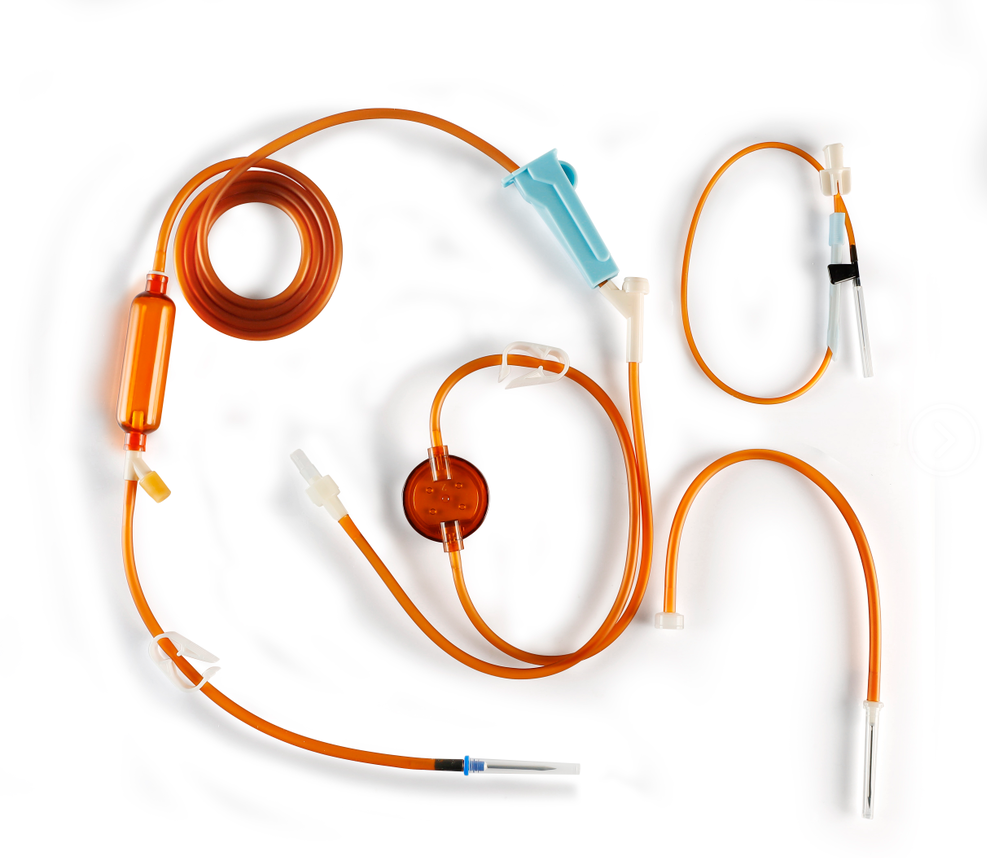
DISPOSABLE OPAQUE INFUSION SETS
Overview:
◆ The inner material of the hose and dripper in contact with the liquid is thermoplastic polyurethane (TPU), which does not contain DEHP; the outer material of the hose and dripper not in contact with the liquid is polyvinyl chloride (PVC) with added light-absorbing agent. The light-avoidance rate of this product in the wavelength range of 290n450m: tubing ≤ 15%, dropper ≤ 35%. The product is sterilized by ethylene oxide, single-use. This product is suitable for the intravenous infusion of levofloxacin, lipoic acid, cyclophosphamide, oxaliplatin, nilmustine, cisplatin, docetaxel, ranitidine, pantoprazole, vitamin C, paclitaxel in the light-avoiding range of 290-450m, and it is limited to gravity infusion.
◆ Safety: It does not contain any harmful substances such as phthalates (HT), vinyl chloride monomer and light-avoiding agents, which prevents the precipitation of these substances from harming patients during infusion.
◆ Extremely wide range of application: In addition to gener
◆ Safety: It does not contain any harmful substances such as phthalates (HT), vinyl chloride monomer and light-avoiding agents, which prevents the precipitation of these substances from harming patients during infusion.
◆ Extremely wide range of application: In addition to gener
Product Features
| Intended use | This product is indicated for the intravenous infusion of levofloxacin, lipoic acid, cyclophosphamide, oxaliplatin, nimustine, cisplatin, docetaxel, ranitidine, pantoprazole, vitamin C, and paclitaxel for gravity infusion only. |
| Structure and composition | The product consists of a stopper puncture device, a puncture device protective sleeve, an air inlet device, a stopcock, a dropper, a dropper, a line, a flow regulator, an injection device, an ordinary medication filter (with a nominal pore size of 15 μm on the membrane) or a precision medication filter (with nominal pore sizes of 2 μm, 3 μm, and 5 μm on the membrane), an external conical connector, a protective sleeve for the external conical connector, and an intravenous infusion needle. |
| Main materials | Thermoplastic polyurethane (TPU), medical polyvinyl chloride PVC, medical polypropylene PP, medical ABS, SUS304 stainless steel tube. |
| Shelf life | 5 years |
| Certification and quality assurance | Manufactured in full compliance with IS013485 quality systems. |
Product Introduction





 Home
Home